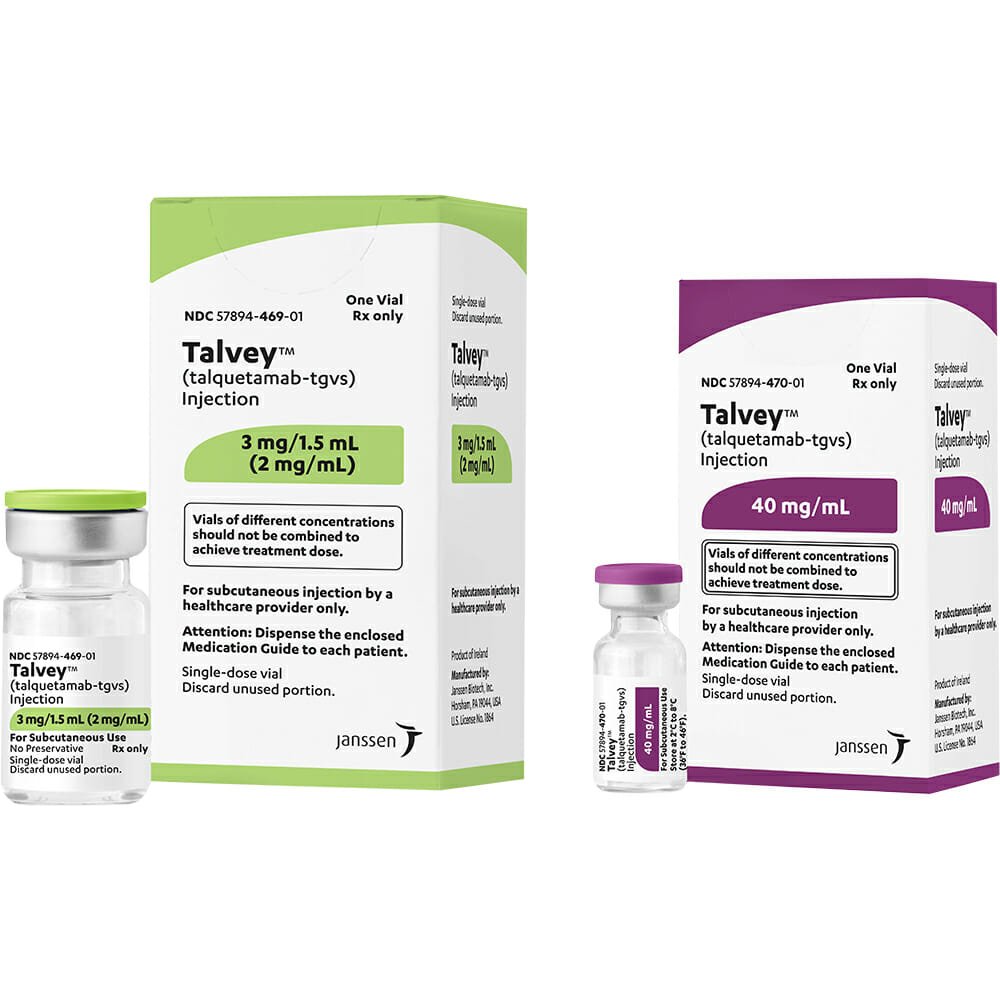
Description
Talquetamab is indicated for the treatment of adults with relapsed or refractory multiple myeloma who have received prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
The most common adverse reactions include cytokine release syndrome, dysgeusia, nail disorder, musculoskeletal pain, skin disorder, rash, fatigue, decreased weight, dry mouth, pyrexia, xerosis, dysphagia, upper respiratory tract infection, and diarrhea.
TALVEY (talquetamab-tgvs) injection, for subcutaneous use Initial U.S. Approval: 2023
See full prescribing information : Click Here
Need Help Getting Medicine? Here’s How We Can Assist You.
Brand Name “TALVEY” or Generic Name “talquetamab-tgvs” can be imported for personal use under “Named Patient Program” treatment in Delhi, Kolkata, Surat, Jaipur, Noida, Gurgaon (Gurugram), Punjab, Chandigarh, Bhubaneswar, Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Tripura and Sikkim, Noida, Kanpur, Lucknow, Dehradun, Shimla, Ahmedabad, Jodhpur, Mumbai, Jaipur, Aurangabad, Pune, Bangalore, Hyderabad, Chennai, Visakhapatnam, Coimbatore, Andhra Pradesh, Karnataka, Kerala, Lakshadweep, Puducherry, Tamil Nadu, Telangana, India. Contact us at support@southdelhipharma.net, southdelhipharma@gmail.com or you can call at 9891296838 or WhatsApp at 9891296838

![REVLIMID [lenalidomide] capsules cost Price Delhi India](https://southdelhipharma.com/wp-content/uploads/2021/01/revlimid-300x300.jpg)
