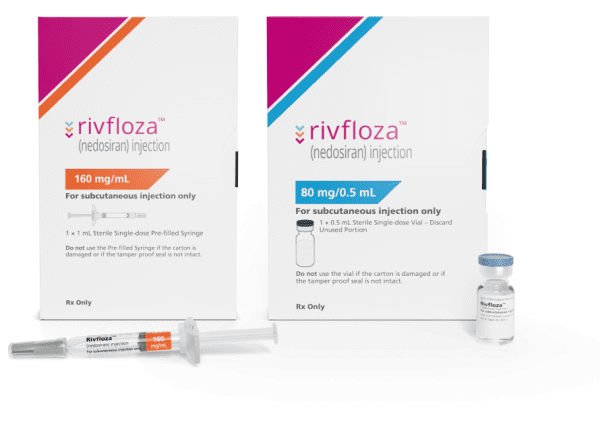
Description
Primary hyperoxaluria type 1 is a rare disease in which urine oxalate is too high, which over time can harm the kidneys. Nedosiran is indicated to lower urinary oxalate levels in people nine years of age and older with primary hyperoxaluria type 1 and relatively preserved kidney function.
The most common side effects include injection site reactions.
RIVFLOZA® (nedosiran) injection, for subcutaneous use Initial U.S. Approval: 2023
See full prescribing information : Click Here
Need Help Getting Medicine? Here’s How We Can Assist You.
Brand Name “RIVFLOZA” or Generic Name “nedosiran” can be imported for personal use under “Named Patient Program” treatment in Delhi, Kolkata, Surat, Jaipur, Noida, Gurgaon (Gurugram), Punjab, Chandigarh, Bhubaneswar, Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Tripura and Sikkim, Noida, Kanpur, Lucknow, Dehradun, Shimla, Ahmedabad, Jodhpur, Mumbai, Jaipur, Aurangabad, Pune, Bangalore, Hyderabad, Chennai, Visakhapatnam, Coimbatore, Andhra Pradesh, Karnataka, Kerala, Lakshadweep, Puducherry, Tamil Nadu, Telangana, India. Contact us at support@southdelhipharma.net, southdelhipharma@gmail.com or you can call at 9891296838 or WhatsApp at 9891296838