Description
Donanemab is indicated for the treatment of Alzheimer’s disease for people with mild cognitive impairment or mild dementia stage of disease.
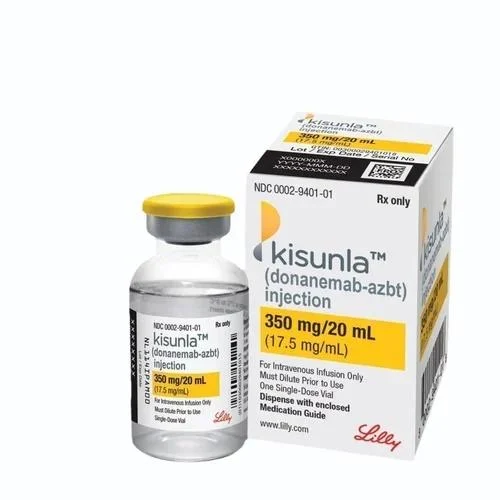
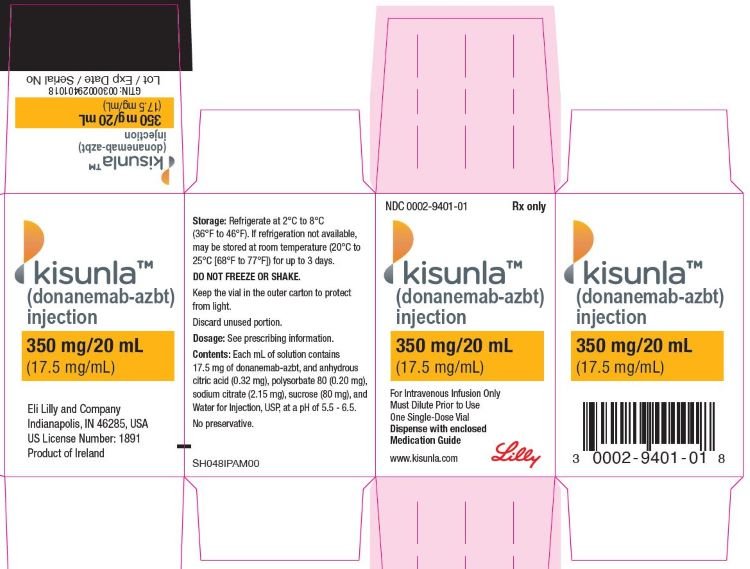
Donanemab was approved for medical use in the United States in July 2024. Most of the members of the FDA advisory panel had financial conflicts of interest. Treatment is intended for people with mild cognitive impairment or mild dementia stage of disease, which is the same population the treatment was studied in the clinical trials. Several public interest groups spoke out in FDA hearings against approval of the drug.KISUNLA (donanemab-azbt) injection, for intravenous use Initial U.S. Approval: 2024
See full prescribing information: Click Here
Get Access To Kisunla (donanemab-azbt) Injection In India on request
Brand Name “Kisunla” or Generic Name “donanemab-azbt” can be imported for personal use under “Named Patient Program” treatment in Delhi, Kolkata, Surat, Jaipur, Noida, Gurgaon (Gurugram), Punjab, Chandigarh, Bhubaneswar, Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Tripura and Sikkim, Noida, Kanpur, Lucknow, Dehradun, Shimla, Ahmedabad, Jodhpur, Mumbai, Jaipur, Aurangabad, Pune, Bangalore, Hyderabad, Chennai, Visakhapatnam, Coimbatore, Andhra Pradesh, Karnataka, Kerala, Lakshadweep, Puducherry, Tamil Nadu, Telangana, India. Contact us at support@southdelhipharma.net, southdelhipharma@gmail.com or you can call at 9891296838 or WhatsApp at 9891296838