Description
Durvalumab is an immune checkpoint inhibitor drug. It was approved in for medical use in the United States in May 2017, and in the European Union in September 2018.
The US Food and Drug Administration (FDA) approved durvalumab for certain types of bladder, lung, and biliary tract cancer:
1. Adults with locally advanced or metastatic urothelial carcinoma who either have disease progression during or following platinum-containing chemotherapy or have disease progression within twelve months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
2. Adults with unresectable, Stage III non-small cell lung cancer whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
3. In combination with etoposide and either carboplatin or cisplatin, as first-line treatment for adults with extensive-stage small cell lung cancer.
4. In combination with gemcitabine and cisplatin for adults with locally advanced or metastatic biliary tract cancer (BTC).
In June 2024, the US FDA approved durvalumab with carboplatin plus paclitaxel, followed by single-agent durvalumab, for adults with primary advanced or recurrent endometrial cancer that is mismatch repair deficient.
In August 2024, the FDA approved durvalumab with platinum-containing chemotherapy as neoadjuvant treatment, followed by single-agent durvalumab as adjuvant treatment after surgery for adults with resectable (tumors ≥ 4 cm and/or node positive) non-small cell lung cancer (NSCLC) and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements
In December 2024, the FDA expanded the indication of durvalumab to include adults with limited-stage small cell lung cancer whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
In March 2025, the FDA approved durvalumab with gemcitabine and cisplatin as neoadjuvant treatment, followed by single agent durvalumab as adjuvant treatment following radical cystectomy, for adults with muscle invasive bladder cancer
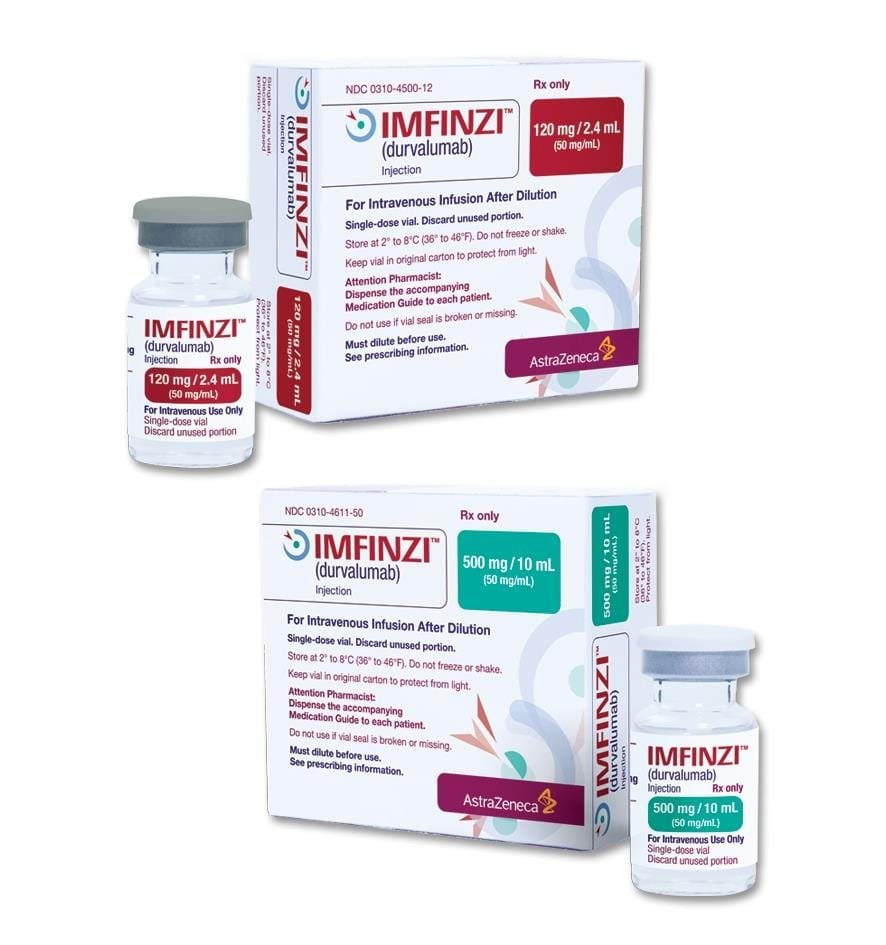
IMFINZI (durvalumab) injection, for intravenous use Initial U.S. Approval: 2017
See full prescribing information : Click Here
Get Access To Imfinzi (durvalumab) Injection In India On Request
Brand Name “Imfinzi” or Generic Name “durvalumab” can be imported for personal use under “Named Patient Program” treatment in Delhi, Kolkata, Surat, Jaipur, Noida, Gurgaon (Gurugram), Punjab, Chandigarh, Bhubaneswar, Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Tripura and Sikkim, Noida, Kanpur, Lucknow, Dehradun, Shimla, Ahmedabad, Jodhpur, Mumbai, Jaipur, Aurangabad, Pune, Bangalore, Hyderabad, Chennai, Visakhapatnam, Coimbatore, Andhra Pradesh, Karnataka, Kerala, Lakshadweep, Puducherry, Tamil Nadu, Telangana, India. Contact us at support@southdelhipharma.net, southdelhipharma@gmail.com or you can call at 9891296838 or WhatsApp at 9891296838