Description
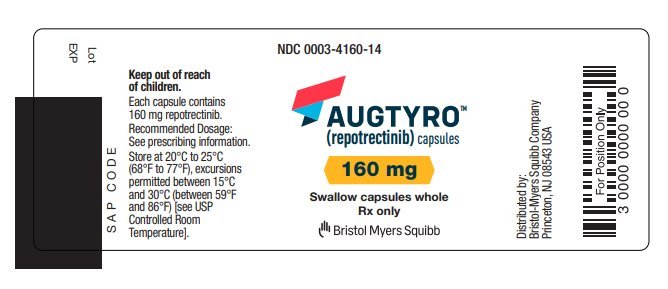
Repotrectinib is indicated for the treatment of adults with locally advanced or metastatic ROS1-positive non-small cell lung cancer.
In June 2024, the US Food and Drug Administration (FDA) expanded the indication to include the treatment of people twelve years of age and older with solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion, are locally advanced or metastatic or where surgical resection is likely to result in severe morbidity, and that have progressed following treatment or have no satisfactory alternative therapy.
Repotrectinib was approved for medical use in the United States in November 2023, and in the European Union in January 2025.
AUGTYRO (repotrectinib) capsules, for oral use Initial U.S. Approval: 2023
See full prescribing information : Click Here
Get Access To Augtyro (Repotrectinib) Capsules In India On Request
Brand Name “Augtyro” or Generic Name “Repotrectinib” can be imported for personal use under “Named Patient Program” treatment in Delhi, Kolkata, Surat, Jaipur, Noida, Gurgaon (Gurugram), Punjab, Chandigarh, Bhubaneswar, Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Tripura and Sikkim, Noida, Kanpur, Lucknow, Dehradun, Shimla, Ahmedabad, Jodhpur, Mumbai, Jaipur, Aurangabad, Pune, Bangalore, Hyderabad, Chennai, Visakhapatnam, Coimbatore, Andhra Pradesh, Karnataka, Kerala, Lakshadweep, Puducherry, Tamil Nadu, Telangana, India. Contact us at support@southdelhipharma.net, southdelhipharma@gmail.com or you can call at 9891296838 or WhatsApp at 9891296838