Description
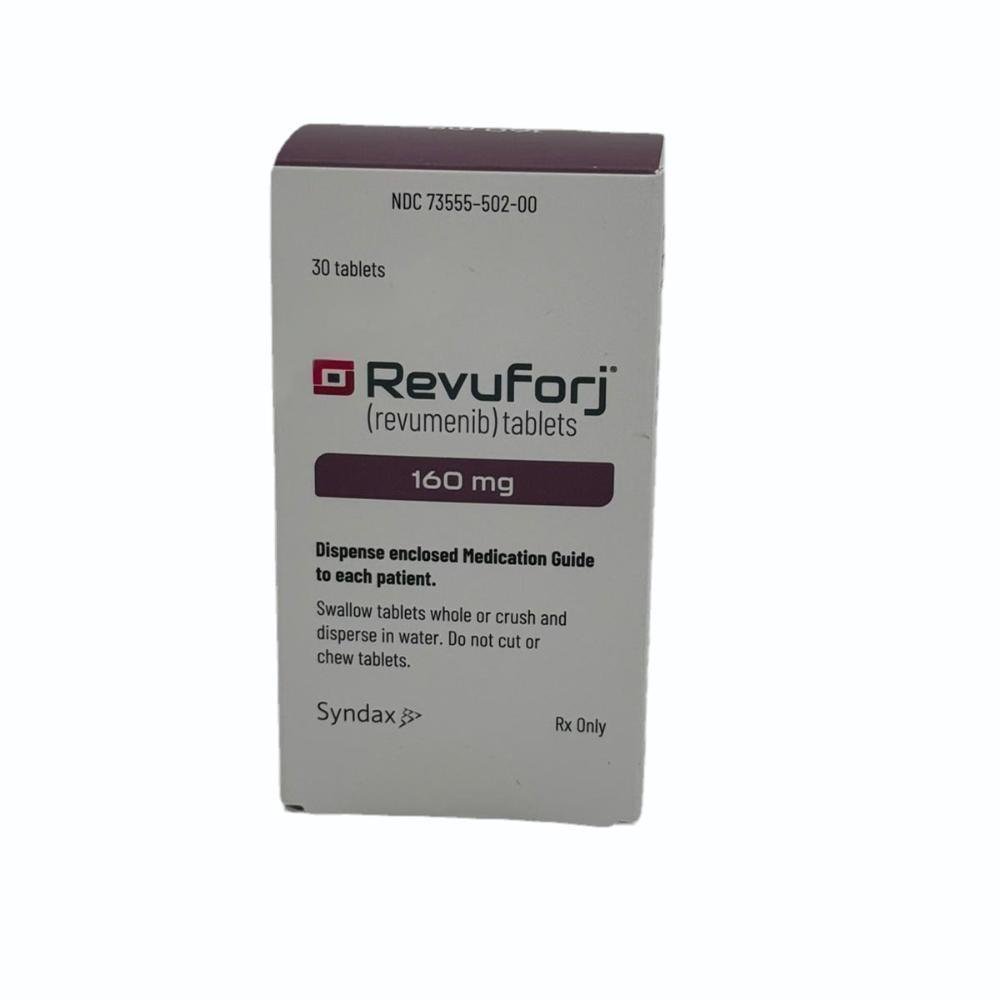
Revumenib is indicated for the treatment of relapsed or refractory acute leukemia with a lysine methyltransferase 2A gene (KMT2A) translocation.
Revumenib was approved for medical use in the United States in November 2024. The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.
REVUFORJ (revumenib) tablets, for oral use Initial U.S. Approval: 2024
See full prescribing information: Click Here
Get Access To Revuforj (revumenib) Tablets In India On Request
Brand Name “Revuforj” or Generic Name “revumenib” can be imported for personal use under “Named Patient Program” treatment in Delhi, Kolkata, Surat, Jaipur, Noida, Gurgaon (Gurugram), Punjab, Chandigarh, Bhubaneswar, Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Tripura and Sikkim, Noida, Kanpur, Lucknow, Dehradun, Shimla, Ahmedabad, Jodhpur, Mumbai, Jaipur, Aurangabad, Pune, Bangalore, Hyderabad, Chennai, Visakhapatnam, Coimbatore, Andhra Pradesh, Karnataka, Kerala, Lakshadweep, Puducherry, Tamil Nadu, Telangana, India. Contact us at support@southdelhipharma.net, southdelhipharma@gmail.com or you can call at 9891296838 or WhatsApp at 9891296838