Description
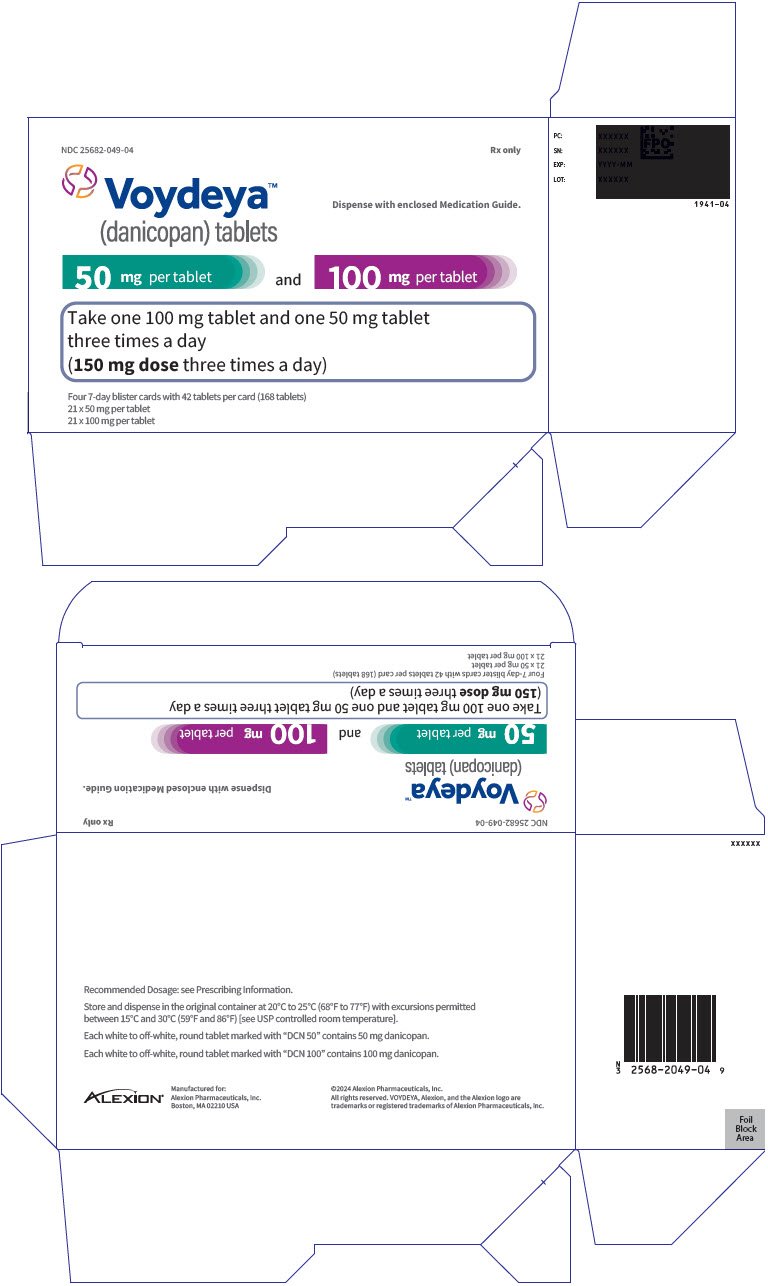
Danicopan is indicated as add-on therapy to ravulizumab or eculizumab for the treatment of extravascular hemolysis in adults with paroxysmal nocturnal hemoglobinuria.
The most common side effects include fever, headache, increased levels of liver enzymes (a sign of possible liver problems) and pain in the extremities (arms and legs).
Danicopan was approved for medical use in Japan in January 2024, in the United States in March 2024, and in the European Union in April 2024. The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.
VOYDEYA™ (danicopan) tablets, for oral use Initial U.S. Approval: 2024
See full prescribing information : Click Here
Get Access To Voydeya (danicopan) Tablets In India On Request
Brand Name “Voydeya ” or Generic Name “danicopan” can be imported for personal use under “Named Patient Program” treatment in Delhi, Kolkata, Surat, Jaipur, Noida, Gurgaon (Gurugram), Punjab, Chandigarh, Bhubaneswar, Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Tripura and Sikkim, Noida, Kanpur, Lucknow, Dehradun, Shimla, Ahmedabad, Jodhpur, Mumbai, Jaipur, Aurangabad, Pune, Bangalore, Hyderabad, Chennai, Visakhapatnam, Coimbatore, Andhra Pradesh, Karnataka, Kerala, Lakshadweep, Puducherry, Tamil Nadu, Telangana, India. Contact us at support@southdelhipharma.net, southdelhipharma@gmail.com or you can call at 9891296838 or WhatsApp at 9891296838